Manufacturers & Suppliers
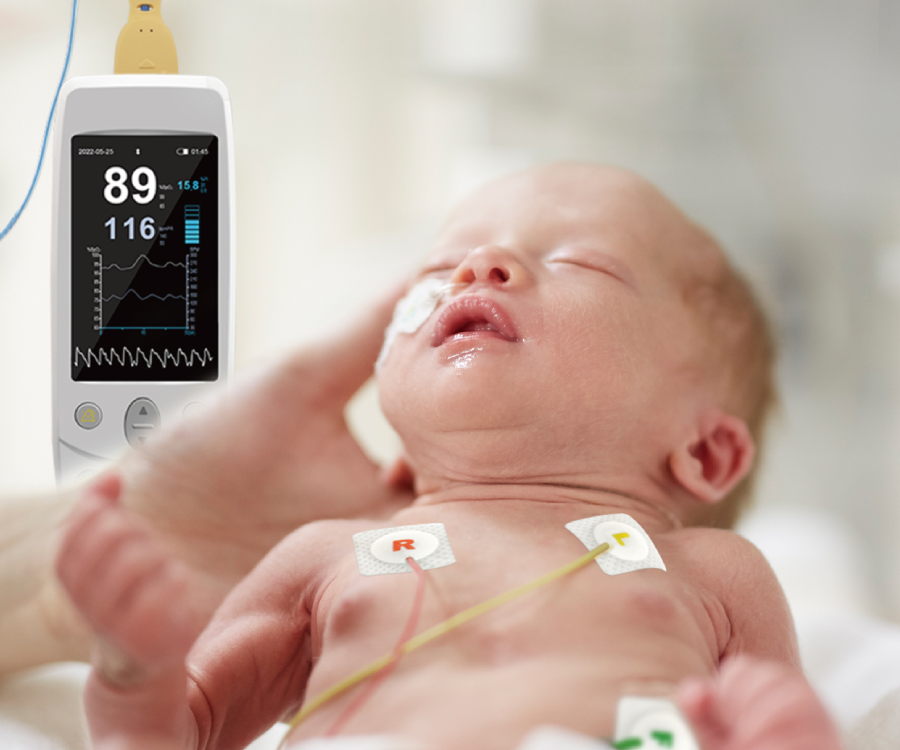
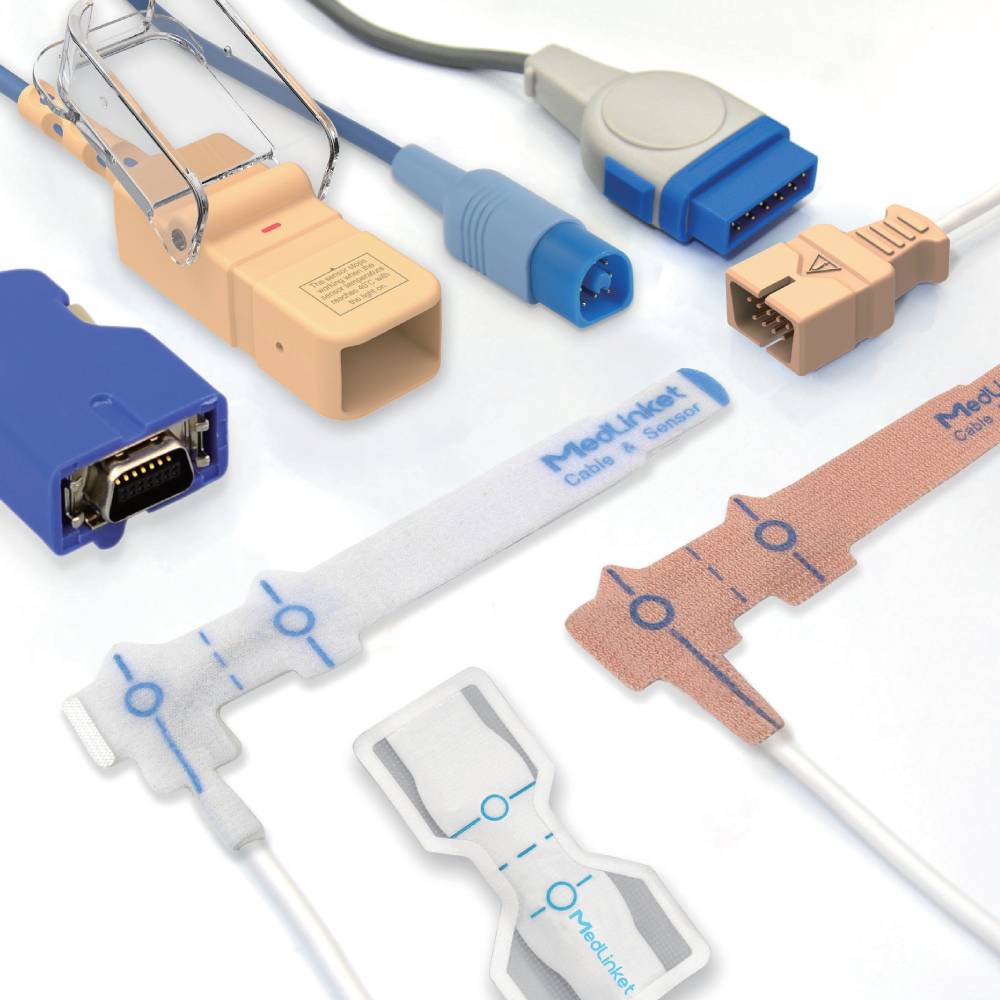
SpO₂ sensors
We provide a full range of SpO₂ sensor sizes for adults, pediatrics, infants and neonates, suitable for different testing positions.


years of experience
about us
“Over 20 Years of SpO₂ Cable Manufacturer
First listed company in patient monitoring consumables in China;
20+ years specializing in medical monitoring consumables;
Products exported to 118+ countries and regions;
2000+ hospital end-users;
The first Chinese manufacturer offering the integrated solution of SpO2/PR/RR/CtHb/MetHb/COHb sensors, cables, modules, and clinical consulting services.
Why Choose Us
Core Advantages
Focus on providing cost-effective medical sensors and cable assemblies to brand owners!

1.
Transaction security
Listed on the New Third Board with a focus on reputation.
Strong capital ensures sustainable development and stable operations.
Backed by $5 million in product liability insurance to mitigate customer risks;

2.
Flexible Manufacturer
Medical sensors come in a wide variety of types and small batches.
Whether it’s a small or large order, we can handle it efficiently.

3.
High Quality
SpO₂ products are our company’s core products, and we provide highly precise clinical comparison reports with the gold – standard “blood gas analysis”.
4.
Get Satisfied with the services we provide A to Z in cleaning
Many SpO2 sensors, cables, molds, etc., have high R&D costs and long cycles.
Our company owns mold, cable, and assembly factories, saving R&D time and mold costs.
R&D Capabilities
1.High Quality R&D Team
Our R & D team is made up of professional and experienced members with excellent problem – solving and innovative skills.

2.Precision R & D Facilities
We have comprehensive facilities, including full – scale physicochemical lab, two sterile labs, two positive control labs, and two microbiology labs.
Our R & D team draws on diverse medical device samples, such as patient monitors, pulse oximeters, ECG machines, and electrosurgical units.
We are equipped with a wide range of testing instruments, including optical testers with wide wavelength ranges, electrical and physical property testers. Professional design software is utilized to ensure high – quality design.

3.R & D Achievements
Our product has secured multiple patents across utility models, design patents, invention patents, and other fields, and has been recognized by renowned domestic and international bodies like the FDA, ANVISA, and MHRA.
4 Confidentiality measures
Security guards are on duty 24 hours a day;
Multiple video surveillance systems are running simultaneously to avoid overstepping of duties and embezzlement;
Network watchdogs and firewalls are installed at the physical network gateways;
All computers are equipped with encryption software from well-known companies;
All employees have signed a strict confidentiality agreement;
All material suppliers have signed a confidentiality agreement





Each item must be qualified before shipment, 100% Qualtiy Test control
In 2005, the company has passed the ISO 13485 quality management system certification, and continues to maintain ISO 13485 and ISO 9001 quality management system certification till this day;
he company has also passed on-site audits by regulatory agencies such as China’s NMPA, the United States FDA, and Brazil’s ANVISA;
Every year, many top patient monitor companies conduct quality management system assessments on MedLinket, and all successfully passed.
150+ Certification
Contact Us
Send Your Inquiry Today
Provide full product process services:consultation, design, research,development, production
WHAT APP
+872 57428634